We have already considered simple Redox reactions and a not so simple Disproportionation Reactions. Those could be easily balanced and parameterized by means of Electrochemistry tool. But what if there are more than two basic redoxes take place within single reaction (let alone non-conventional redox states…) ? Here we will consider a good example by balancing a following beast:
Cr7N66H96C42O24 + MnO4– + H+ = Cr2O72- + Mn2+ + CO2 + NO3– + H2O
Lets start from the obvious:
- Reaction takes place at acidic conditions (+ H+).
- We already can spot the first reduction half-reaction:
MnO4– = Mn2+
which is easily balanced in acidic conditions to:
MnO4– + 8H+ + 5e– = Mn2+ + 4H2O R1
But what happens with Cr7N66H96C42O24 ??? We have to know oxidation states before any attempt to balance redox reaction. The actual structure of compound would be really helpful for this matter:
[Cr(N2H4CO)6]4[Cr(CN)6]3
Quite complex compound consisting of two ionic complexes: Chromium (III) Hexaurea [Cr(N2H4CO)6]3+ and Chromium (II) Hexacyanide [Cr(CN–)6]4- .
Apparently, there are chromes in two different oxidation states, that all will end up in Cr2O72- , where chrome has oxidation state of +6. And then, oxidation state of Nitrogen, both in urea and in cyanide anion (CN–) starts from -3 and goes to +5 in NO3–. Carbon, on the other hand, starts from +4 in N2H4CO (doesn’t change the state) and +2 in CN– and goes to +4 in CO2 . It would be even funnier, if the same element would be getting different oxidations states in products. But let’s not exaggerate!
So we got 1 reduction half-reaction (R1) and four oxidations. All half- reactions are balanced with acidic condition in mind, so only H+ and H2O can be added to reactants to balance hydrogen and oxygen.
MnO4– + 8H+ + 5e– = Mn2+ + 4H2O R1
2 Cr3+ + 7 H2O = Cr2O72- + 14 H+ + 6 e– R2
N2H4CO + 7 H2O = CO2 + 2 NO3–+ 18 H+ + 16 e– R3
2 Cr2+ + 7 H2O = Cr2O72- + 14 H+ + 8 e– R4
CN– + 5 H2O = CO2 + 10 H+ + NO3– + 10 e– R5
* Important to understand that R2 and R3 are from [Cr(N2H4CO)6]3+ and R4 and R5 are from [Cr(CN–)6]4-. But!!! all these compounds are from the same molecule and it’s important to maintain the ratios between them to match the ratios in the original complex.
In [Cr(N2H4CO)6]3+ Cr to urea ratio is 1 to 6, but in R2, chromes stoichiometric coefficient is 2, so R3 must be taken 12 times to match the ratio:
R6 = 12*R3 + R2
Chemical Reactions app can come in handy for these kind of calculations:

2Cr3+ + 91H2O + 12N2H4CO = 12CO2 + Cr2O72- + 230H+ + 24NO3– + 198e–, R6
Similarly, in [Cr(CN–)6]4- Cr to CN–ratio is 1 to 6, but in R4, chromes stoichiometric coefficient is 2, so R5 must be taken 12 times to match the ratio:
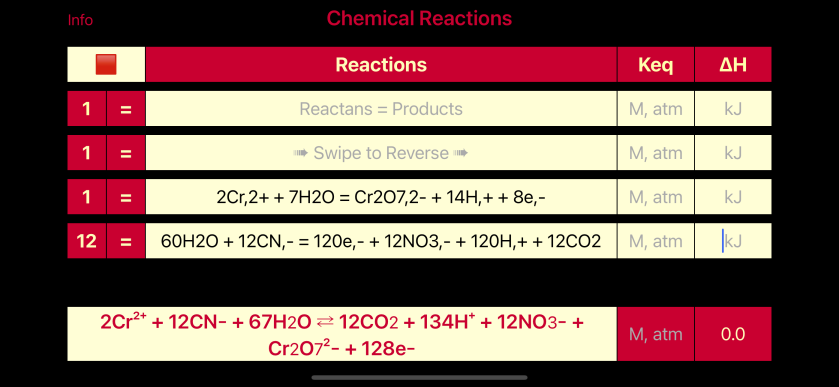
R7 = 12*R5 + R4
12 CN– + 2 Cr2+ + 67 H2O = 12 CO2 + Cr2O72- + 134 H+ + 12 NO3– + 128 e–, R7
Ratio between [Cr(N2H4CO)6]3+ and [Cr(CN–)6]4- in the original complex is 4 to 3. Lets see what we have now if we rewrite R6 and R7 equations:
2[Cr(N2H4CO)6]3++ 91H2O = 12CO2 + Cr2O72- + 230H+ + 24NO3– + 198e– , R6′
2[Cr(CN–)6]4-+ 67 H2O = 12 CO2 + Cr2O72- + 134 H+ + 12 NO3– + 128 e– , R7′
The curent ratio is 2:2, so if need 4:3, then:
R8 = 4*R6 + 3*R7
36 CN– + 6 Cr2+ + 8 Cr3++ 565 H2O + 48 N2H4CO = 84 CO2 + 7 Cr2O72- + 1322 H+ + 132 NO3– + 1176 e– , R8
or
2[Cr(N2H4CO)6]4[Cr(CN)6]3 + 565 H2O = 84 CO2 + 7 Cr2O72- + 1322 H+ + 132 NO3– + 1176 e– , R8′
R8 is a half-reaction of oxidation! Now in the final step lets combine oxidation with reduction, while balancing the electrones:
Rfinal = 5* R8 + 1176*R1

Enough for now….