Solubility Products is a scientific application available on Play Store, Apple Store for iOS and Mac, that helps with accurate estimation of concentrations of solutes upon dissolution of solid compounds of low solubility. The tool is applicable to various chemical equilibrium tasks, such as salt dissociation to electrolytes, common-ion effect, separation by sedimentation and precipitation titration. Application derives final, equilibrium concentrations of solutes based on equilibrium constant (Ksp), initial concentrations, solubility and stoichiometric coefficients. Additionally, app calculates reaction quotient for any given concentrations and stoichiometry.
To start the calculation the following data is required: balanced dissociation reaction equation, initial concentrations of components, solution volume and equilibrium constant Ksp or Solubility.

The solubility in pure solvent (water) only is evaluated and filled in and not in the current conditions. Presence of common ions can decrease the solubility!
The application provides database of low solubility compounds. Upon selection of the suggested compound, app fills in dissociation reaction and equilibrium constant.

H2O molecules released upon dissolution of hydrates are not taken into consideration, since water is a medium where reaction takes place.
Either Ksp or Solubility values must be present! When only solubility value is available the app will automatically calculate Ksp and vice versa.
Volume of solution is a must to evaluate change in amount of solid compound, it is adjustable and automatically set to 1 litre.
Important!!! If available amount of solid is not enough, then it will be completely dissolved without achieving equilibrium conditions!
When Molar mass (Mw) of solid is filled in, then solid mass and solubility calculated and inserted in g/l units, otherwise it is set to mole/l. Beware of transformations accompanied with filling and deleting of Mw value! Once Mw value is filled in and “Done” is pressed (!), a solid mass and solubility are recalculated to new units.

To start calculation initially or after updating the concentration or coefficient fields, user is requested to tap Run button! The values are valid only if Run button is greyed out!
Significant attention should be paid to concentration, solubility and equilibrium constant units!
The power of equilibrium constant units depicted as “n” are defined as (a+b+c), where a, b and c are the stoichiometric coefficients of the soluble dissolution products.
The Theoretical Background
For a general dissociation chemical reaction:
S(s) ⇔ aA + bB +cC
the Reaction Quotient (Q) is defined by:
where A, B, C – are solutes (electrolytes, ions, etc.) concentrations and a, b, c are stoichiometric coefficient. Sum of powers determines the reaction order.
If Q is not equal to reaction equilibrium constant (Ksp), then reaction is not at equilibrium and it will proceed (due to difference in forward and reverse reaction speeds) to the direction defined by Q.
If Q < Ksp, reaction will proceed toward products (A&B&C), otherwise if Q > Ksp, reverse reaction will prevail.
Example:
For given reaction: S(s) <=> 2A + 1B +3C, initial reaction concentrations are Ao=0.1M, Bo=0.2M, Co=0.3M and Ksp=1e-6 M6. Reaction Quotient (Q) is defined by:
Upon loading the data to “Solubility Products” app, Reaction Quotient is immediately available: Q= 5.4e-5 M6. Apparently Q>K, suggesting that reaction is too close to the products side and reverse reaction (precipitation) will prevail to bring the reaction back to the equilibrium. The new equilibrium concentrations of reaction components can be derived from the equation:
Apparently, solving this equation is rather demanding task, that turns to be unnecessary, since “Solubility Products” app immediately returns the answer: Aeq=28.98mM, Beq=0.1645M, Ceq=0.1935M and additionally 0.036 mole of solid precipitated.



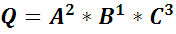


2 thoughts on “Solubility Products”
Comments are closed.